
It is in the same proportion to the atom as a marble is to a football field. Compared with the overall size of the atom, the nucleus is even more minute. A convenient unit of length for measuring atomic sizes is the angstrom (Å), defined as 10 −10 metre. Approximately 50 million atoms of solid matter lined up in a row would measure 1 cm (0.4 inch).
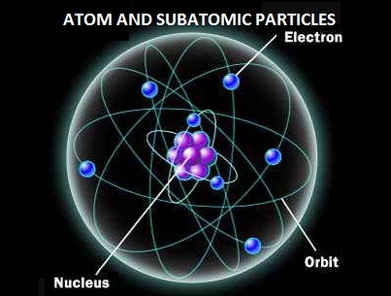
They can be created only with the addition of enormous amounts of energy, however, and are very short-lived.Īll atoms are roughly the same size, whether they have 3 or 90 electrons. Other subatomic particles may be found in association with these three types of particles. Protons, neutrons, and the electrons surrounding them are long-lived particles present in all ordinary, naturally occurring atoms. It is composed of protons, which have a positive charge, and neutrons, which have no charge. The nucleus is the positively charged centre of an atom and contains most of its mass. Attempts to separate these smaller constituent particles require ever-increasing amounts of energy and result in the creation of new subatomic particles, many of which are charged.Īs noted in the introduction to this article, an atom consists largely of empty space. These particles are electrically charged, and the electric forces on the charge are responsible for holding the atom together. Each individual atom consists of smaller particles-namely, electrons and nuclei. Molecules, in turn, are composed of atoms joined by chemical bonds that are more difficult to break. Most matter consists of an agglomeration of molecules, which can be separated relatively easily. Get a Britannica Premium subscription and gain access to exclusive content. For additional information pertaining to nuclear structure and elementary particles, see subatomic particles. Following this overview is a historical survey of the most influential concepts about the atom that have been formulated through the centuries. This article opens with a broad overview of the fundamental properties of the atom and its constituent particles and forces. The behaviour of an atom is strongly influenced by these orbital properties, and its chemical properties are determined by orbital groupings known as shells. Such wave patterns, called orbitals, describe the distribution of individual electrons. In others, the electrons behave like waves frozen in position around the nucleus. In some respects, the electrons in an atom behave like particles orbiting the nucleus.
Are atoms the smallest particles of matter how to#


From tech to household and wellness products. This Time in History In these videos, find out what happened this month (or any month!) in history.#WTFact Videos In #WTFact Britannica shares some of the most bizarre facts we can find.Demystified Videos In Demystified, Britannica has all the answers to your burning questions.Britannica Explains In these videos, Britannica explains a variety of topics and answers frequently asked questions.Britannica Classics Check out these retro videos from Encyclopedia Britannica’s archives.


 0 kommentar(er)
0 kommentar(er)
